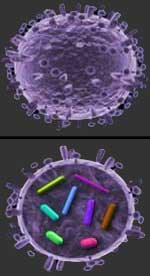
An illustration of the influenza virus from the outside (top), and cut away to reveal the RNA (bottom). Hemagglutinin and neuraminidase are the spikes on the outside of the virus.
By spring of this year, Jeffery Taubenberger expects to have in hand the entire genetic sequence of the influenza virus responsible for 1918’s devastating global epidemic. Taubenberger, a molecular pathologist at the Armed Forces Institute of Pathology in Rockville, Maryland, is using the 1918 flu genome information to answer lingering mysteries about the flu virus, such as where it originated, why it was so virulent, and why it struck with such ferocity among presumably healthy young adults. Already, he and other researchers have come up with some intriguing clues:
The Origin
The influenza A virus, the particular type of influenza that causes illness in humans, occurs naturally and without symptoms or disease in waterfowl like duck and geese. Taubenberger’s analysis of the 1918 strain showed that the hemagglutinin protein, one of two receptor proteins on the surface of the virus capsule which helps it gain entry into host cells, was similar to the proteins of bird flu strains, but yet was not identical. That led Taubenberger to suspect that the pandemic virus had either jumped right from birds in 1918 (as happened in the 1957 and 1968 influenza pandemics) and then rapidly mutated, or originally came from a wild bird but spent quite a bit of time in an intermediate host susceptible to flu infection — a domestic chicken, say, or a pig — before making the move to people. Taubenberger eventually ruled out the idea of an intermediate host. He and his colleagues searched for a natural host for the 1918 strain by examining hundreds of ducks and water birds dating from around 1918 that had been preserved in ethanol and stored at the Smithsonian Institution. “We found a number of birds that were positive in our molecular analysis for influenza, but the genes looked basically identical to those isolated from modern birds,” Taubenberger says, and none looked like the killer 1918 virus, which makes a rapidly changing bird flu virus an unlikely source of the pandemic bug. “Our current theory is that the 1918 virus derived from an animal source of influenza that has not yet been identified. It could certainly be a bird, but it is not your typical bird flu host, like ducks, geese, and shore birds. It’s still a mystery.”
The Spread
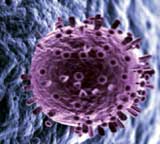
This illustration shows the influenza virus penetrating the wall of a human cell.
The 1918 virus’s virulence — its exceptional ability to infect and swiftly spread through the population — also remains something of a puzzle. Taubenberger and his colleagues have found that one particular virus protein, called NS1, seemed to increase the virulence of the bug by temporarily dampening the host’s immune system, so the virus can get into cells, replicate, and spread through the body. In early February, British researchers reported uncovering another potential cause for the virus’s deadly efficiency. Using Taubenberger’s gene sequence, the team recreated the hemagglutinin protein and analyzed its structure. They found that the protein had been slightly altered compared to similar bird flu strains, a modification that allowed it to bind particularly well to human cells.
The Deadly Toll
Unfortunately, the sequences of the 1918 flu genes offer no explanation for why the most deaths in the pandemic were among young adults, 18-35 years old — a population that traditionally has the lowest influenza death rate. Taubenberger suspects that the virus’s lethality had less to do with its genetic sequence than with an unusual immune response to it in its victims. The idea is that prior exposure to a different strain of virus — an earlier case of the flu — caused their body to react improperly to the 1918 bug, perhaps allowing it to replicate and infect cells and tissues even faster than it normally would have. “It made them particularly susceptible to die,” he says.
“In a way, such an immune response makes a 1918-type pandemic less likely to reappear. If it is not just a feature of the virus, but the virus plus some set of really odd conditions that had to be lined up before this happened, the chances of all those dominoes being lined up in the right order again seems unlikely. But that’s not very satisfying. Obviously, you want a firm answer.”
Other researchers also suspect a unique link between flu and the human immune system, but one that affects the virus, not its human host. Last spring, molecular evolutionist Robin Bush of the University of California at Irvine, biological modeler Neil Ferguson of Imperial College London, and their colleagues published a mathematical model that explains how new strains of influenza appear. Compared with other organisms, the influenza A virus has an odd evolutionary pattern; it mutates rapidly to form new strains, but then nearly all the variants quickly die out, producing a stick-straight evolutionary tree rather than a typical branching bush. Only one strain is dominant at a time, all over the world; other strains can’t get a foothold. The dominant strain can change in a heartbeat, though, which is why vaccine developers have to be diligent about the viral targets of the shots. Bush and her colleagues found that the unique pattern could only be explained if the human immune system plays a key role. “The only thing that produced this pattern in our model was to have a generalized host immune response that did not let you get any kind of flu for a couple of weeks after you had it,” she says. New strains that arise during the outbreak therefore have no one to infect, and die out. Although immunologists have no clear evidence that the immunity exists — or any idea of how it works — the theory has fabulous potential; exploiting that response could someday lead to new types of super-vaccines that, for example, would ramp up the immune system to maintain that short-term protection over the long haul to completely stave off the flu.
These photos show the flu vaccine under development, and the usual method of vaccination.
Such vaccines, if even possible, are at least decades in the future, and that doesn’t help flu specialists now faced with the looming threat of another pandemic like 1918’s. “Nobody can say with certainty that there will be another pandemic, but if you go back several hundred years in history it looks like, on average, a pandemic emerges every 30 years,” Taubenberger says. The last pandemic was in 1968 — 36 years ago. “We can’t fully predict the future, but I think it is quite likely that a pandemic will occur again just because they have occurred in the past. What also makes it more likely is that we have more intense agricultural practices, these megafarms where you have many thousands of animals in close proximity, which makes it easier for the virus to get into animals and spread” — both within species and to other susceptible species, like humans.
Nor does the promise of a super-vaccine help researchers deal with the next flu season. During this past flu season, the influenza vaccine didn’t quite hit the mark; the flu that raged through the winter was a new, or “drifted,” strain not targeted by the vaccine. According to a preliminary study by the Centers for Disease Control of vaccinated healthcare workers in Colorado, the vaccine was not effective or had very low effectiveness against “influenza-like illness” in a group of healthcare workers in Colorado. “The problem was one of mechanics,” says Robin Bush. “There is a huge time lag between when the strain selection is made in February and upcoming flu season. It takes from then until the fall to develop the virus, grow it in culture, get FDA approval, etc. And during that lag time, our summer, it is flu season in the southern hemisphere and the strain can change.”
“The surveillance network that exists, founded by the World Health Organization, is really excellent at looking at influenza in humans and identifying strains that are mutants from the dominant one and might emerge in the next year or two as the one they want to base a vaccine on,” Taubenberger says. “The decisions are difficult but nine out of ten times they get it right. More surveillance, more data, would be good, but there also should be increased surveillance at the animal-human interface, of people who interact with animals at live food markets in Southeast Asia, or farmers who are involved with animals that can be host to the flu. We need to see how often these people are exposed to influenza virus, what the rate of infection is, etc, because it looks like these pandemic viruses form when influenza viruses move from animals into humans. It’s an economic issue too, and there are limited resources, but I think that influenza really needs to be thought of as a major public health problem, and it normally is not. People use the term ‘flu’ rather lightly and equate it with a cold, although just a normal flu makes you really ill.”



